NKX019: Engineered to Target CD19
There remains a substantial unmet medical need today for patients with many B cell-mediated diseases. NKX019 targets the CD19 antigen, a cell surface protein expressed on all types of B cells, including naïve, memory and plasmablast lineages.
In autoimmune disease, B cells can produce antibodies that impede or damage the function of healthy, normal cells. With NKX019, our goal is to target and eliminate these pathogenic B cells that are believed to underpin multiple autoimmune diseases.
Autoimmune disease represents a major unmet need in the United States, with 7 million people afflicted with a B cell-mediated autoimmune disease.
Biologics and small molecule inhibitors targeting inflammatory cytokines, immune cells and intracellular kinases have become the standard-of-care to treat autoimmune diseases.
Immunosuppressants, including corticosteroids, are also prescribed to prevent the immune system from attacking healthy cells and tissues. However, their benefit is limited and requires patients to receive lifelong treatment — which often results in serious side effects.
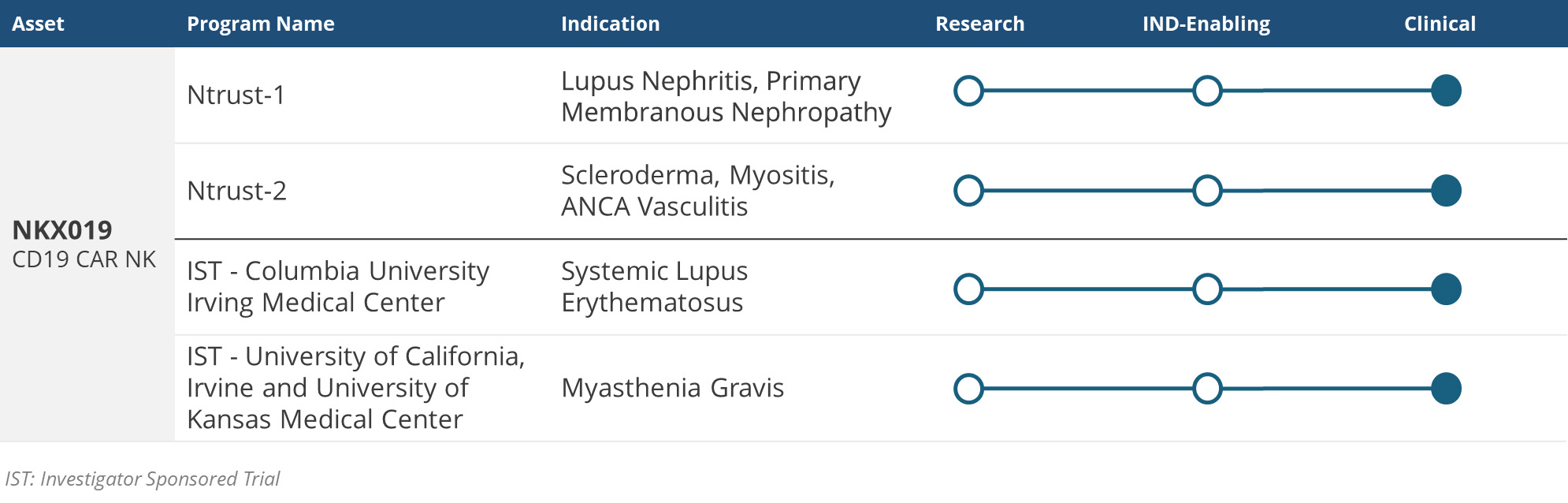
Ntrust-1, Nkarta’s multicenter Phase 1 clinical trial of NKX019 for the treatment of lupus nephritis has initiated dosing and is currently enrolling patients. Please visit Ntrust-1.com or ClinicalTrials.gov for more information (NCT06557265).
Ntrust-2, Nkarta’s multicenter Phase 1 clinical trial of NKX019 for the treatment of systemic sclerosis, inflammatory myopathy and antineutrophil cytoplasmic antibody-associated vasculitis, is currently enrolling patients. Please visit Ntrust-2.com or ClinicalTrials.gov for more information (NCT06733935).
A Phase 1 investigator-sponsored trial of NKX019 for the treatment of systemic lupus erythematosus has initiated dosing and is currently enrolling patients. Please visit ClinicalTrials.gov for more information (NCT06518668).
A Phase 1 investigator-sponsored trial of NKX019 for the treatment of patients with myasthenia gravis is currently enrolling patients.
Preliminary data from our Phase 1 hematological malignancy clinical trials supports the safety and tolerability of CAR-NK cells, with no cases of cytokine release syndrome (CRS), immune cell-associated neurotoxicity syndrome (ICANS) or graft-versus-host disease (GvHD) higher than Grade 3 reported.