TECHNOLOGY
Allogeneic Natural Killer Cells Engineered to Target Autoimmune Diseases
Our focus is to enhance the power, applicability and tolerability of cell therapy by developing next generation cell therapies that leverage the inherent pathogen fighting power of NK cells.
Engineered and enhanced NK cells
Proprietary expansion technology
Potential to reach more patients
ENGINEERING FOR ENHANCED EFFECTIVENESS
NK cells have intrinsic properties that make them attractive candidates for cell therapies to treat B cell-mediated autoimmune diseases:
- They are designed to act immediately upon administration
- It has a favorable profile that may reduce the need to monitor the patient for toxicities after administration
Through advanced engineering, we aim to enhance the effectiveness and persistence of donor NK cells in the patient’s body with two key features:
- A chimeric antigen receptor (CAR) designed to target CD19, a surface protein expressed by B cells that cause autoimmune disease
- Membrane-bound IL-15, a cytokine (immune-signaling protein) that promotes the activity and persistence of NK cells
These features are engineered intentionally to enable our CAR NK cell therapy to recognize and eliminate B cells without help from other immune cells. This initiates the immune reset that allows normal B cells, but not pathogenic B cells, to replenish.
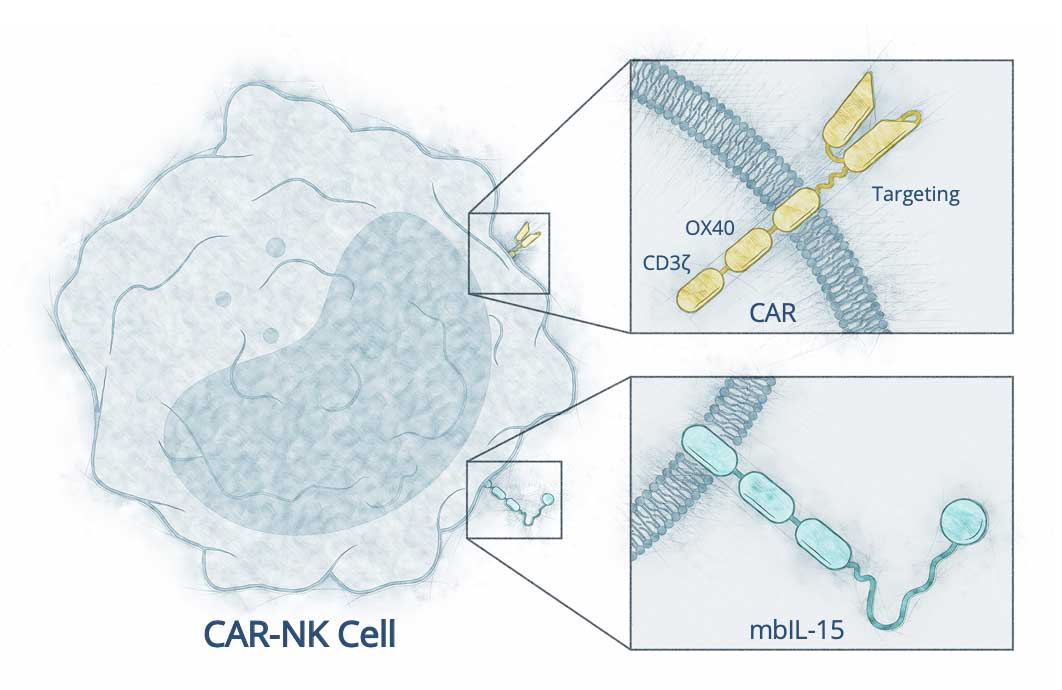
Targeting receptor, OX40 costimulatory
domain, CD3ζ signaling moiety,
membrane bound IL-15
DELIVERING SAFETY ADVANTAGES
Our CAR NK cells are infused at the therapeutic doses intended to eliminate pathogenic B cells. Because they don’t have to expand in the body to be effective, the risk of severe side effects, such as cytokine release syndrome or immune effector cell-associated neurotoxicity syndrome are greatly reduced. Other safety advantages of our CAR NK cell therapies include:
- No risk of causing graft-versus-host disease (GvHD) – a common complication with other allogeneic cell therapies – because NK cells lack the markers that could trigger GvHD
- Clearance from the body in a matter of weeks
INCREASING ACCESS TO THERAPY WITH ALLOGENEIC CAR NK CELLS
Off-the-shelf cell therapy products are ready to infuse as soon as patients need them. NKX019 has the potential to reach a broader patient population for three reasons:
- It is made from healthy donors’ cells, sparing patients the inconvenience of leukapheresis (cell-collecting procedure) and treatment delays associated with autologous cell therapies
- It has a highly favorable profile that reduces the need to monitor the patient for toxicities after administration
- They can be manufactured at scale and made available for outpatient administration
Nkarta’s goal is to offer patients with B cell-mediated autoimmune diseases the life-changing treatment options they deserve by providing effective, safe and accessible CAR NK cell therapies.
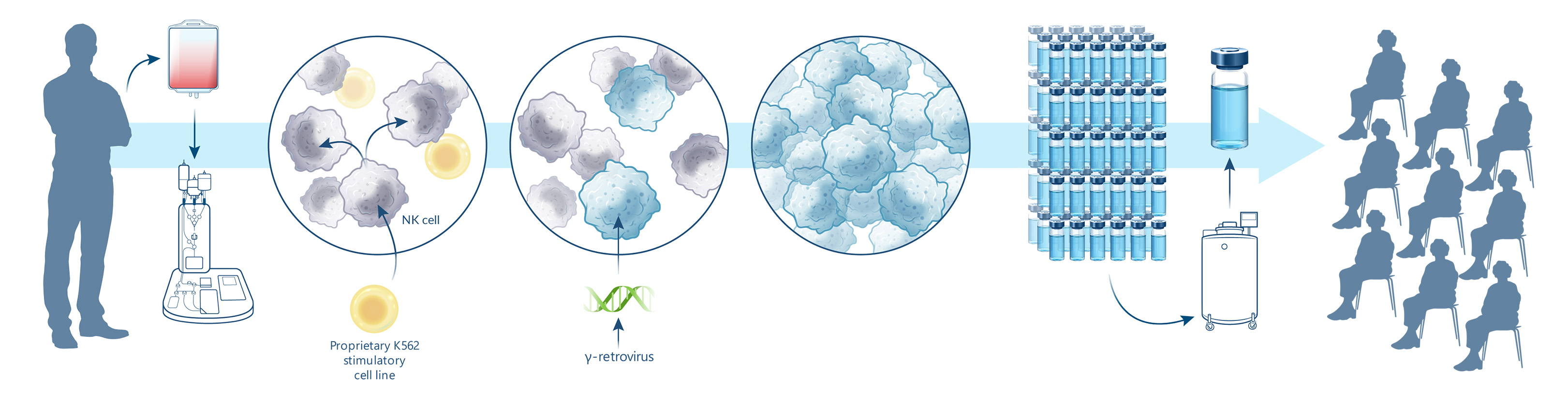
Nkarta’s off-the-shelf Manufacturing From Healthy Donor cells
 |
|||||
|
1 | Collect NK cells are collected from healthy donors by leukapheresis. |
2 | Activate NK cell expansion using our proprietary growth process. |
3 | Engineer Expanded NK cells are genetically programmed to express membrane-bound IL-15 and CAR. |
4 | Expand Continued expansion driven by the introduced membrane-bound IL-15. |
5 | Cryopreserve Harvesting and cryopreservation of final cell product. |
6 | Thaw & Administer NK cells are thawed for off-the-shelf administration to patients at the time they need it. |
